Pharmaceutical Emulsions Industry Set to Reach $2.89B by 2033 | Market Trends & Growth
“Pharmaceutical Emulsions: The Hidden Engine Powering Modern Drug Delivery”
USA Pharmaceutical Emulsions Market to Hit $2.89B by 2033 | Global Trends, Growth Drivers & CAGR 6.4% Forecast 2025-2033”
AUSTIN, TX, UNITED STATES, September 26, 2025 /EINPresswire.com/ -- Market Size:— DataM Intelligence 4Market Research LLP
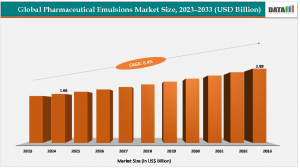
According to DataM Intelligence, the global pharmaceutical emulsions market size reached US$ 1.66 Billion in 2024 from US$ 1.57 Billion in 2023 and is expected to reach US$ 2.89 Billion by 2033, growing at a CAGR of 6.4% during the forecast period 2025-2033.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/pharmaceutical-emulsions-market
Key Market Highlights
• North America leads the pharmaceutical emulsions market, accounting for the largest revenue share of 42.17% in 2024.
• The Asia Pacific region is the fastest-growing market, projected to expand at a CAGR of 6.7% during the forecast period.
• By route of administration, the injectable segment dominated the market, holding the largest revenue share of 56.13% in 2024.
• Prominent players in the pharmaceutical emulsions market include Fresenius Kabi, Baxter, B. Braun SE, Pfizer Inc., Glenmark Pharmaceuticals Ltd., Amneal Pharmaceuticals LLC, Grifols, S.A., Kelun, and Guangdong Otsuka Pharmaceutical Co., Ltd., among others.
The pharmaceutical emulsions market, often described as the hidden engine powering modern drug delivery, is gaining momentum as emulsions become indispensable in critical care, nutrition, and advanced therapeutics. Pharmaceutical emulsions, whether oil-in-water (O/W), water-in-oil (W/O), or multiple emulsions, offer unique advantages such as enhancing the solubility of poorly water-soluble drugs, improving bioavailability, enabling controlled release, and serving as safe carriers for both nutrients and therapeutic agents. In parenteral nutrition, injectable lipid emulsions like Intralipid, SMOFlipid, Clinolipid, and Omegaven play a vital role in supplying essential fatty acids and calories to premature infants, critically ill adults, and surgical patients. Beyond nutrition, emulsions are increasingly being used in anesthesia (Propofol Injectable Emulsion, USP), oncology drug delivery, and antibiotic formulations, expanding their therapeutic applications.
Moreover, the emergence of nanoemulsions and lipid nanoparticles is redefining the scope of pharmaceutical emulsions, particularly in delivering targeted therapies, biologics, and lipophilic drugs. Despite challenges like stability and shelf-life limitations, the market is supported by robust R&D pipelines and investments from major players like Fresenius Kabi, Baxter, and B. Braun, ensuring a continuous stream of innovation. With rising chronic disease prevalence, aging populations, and ICU admissions globally, pharmaceutical emulsions are poised to remain a cornerstone in modern drug delivery, bridging the gap between therapeutic innovation and patient survival.
Major Companies:
Major companies working towards the market's growth include Fresenius Kabi, Baxter, B. Braun SE, Pfizer Inc., Glenmark Pharmaceuticals Ltd., Amneal Pharmaceuticals LLC, Grifols, S.A., Kelun, and Guangdong Otsuka Pharmaceutical Co., Ltd., among others.
Get Customization in the report as per your requirements:- https://www.datamintelligence.com/customize/pharmaceutical-emulsions-market
Regional Analysis
North America: North America leads the global pharmaceutical emulsions market, holding a 42.17% share in 2024. The region’s dominance is fueled by major market players, innovative product launches, FDA approvals, high healthcare spending, and strong adoption of advanced therapies. The U.S., in particular, drives growth with a dense network of hospitals, NICUs, and critical care units relying on injectable lipid emulsions for parenteral nutrition in critically ill patients and preterm neonates. FDA-approved products like SMOFlipid and Clinolipid exemplify widespread clinical acceptance.
For example, in April 2025, Avenacy launched Propofol Injectable Emulsion USP as a generic equivalent to Diprivan for anesthesia and sedation in adults and pediatric patients. Similarly, in August 2024, Amneal Pharmaceuticals received FDA approval for Propofol Injectable Emulsion USP single-dose vials, reinforcing the region’s strong regulatory support and market growth.
Asia Pacific: The Asia Pacific is the fastest-growing pharmaceutical emulsions market, with a projected CAGR of 6.7% in 2024. Rapid urbanization, expanding healthcare infrastructure, rising disposable incomes, and increasing ICU and neonatal care facilities are driving demand. Countries like China, India, Japan, and South Korea are witnessing higher adoption of lipid emulsions for parenteral nutrition and critical care therapies.
Major players such as Fresenius Kabi, Baxter, and B. Braun are expanding in APAC through product launches and strategic partnerships, introducing products like SMOFlipid and Omegaven in select markets. Growing chronic disease prevalence, aging populations, and rising ICU admissions further fuel market opportunities, making APAC a highly lucrative region.
Europe: Europe’s pharmaceutical emulsions market is experiencing steady growth, driven by rising demand for parenteral nutrition and critical care therapies. The region benefits from strong healthcare infrastructure, regulatory approvals from the EMA, and innovations in lipid-based drug delivery systems. Products like SMOFlipid and Clinolipid are widely used in neonatal and critical care units, supporting both nutritional and therapeutic applications.
Europe is also innovating with drug-loaded fat emulsions and lipid nanoparticles, enhancing drug solubility, bioavailability, and targeted delivery for chemotherapy, antibiotics, and anesthesia. Combined with clinical necessity, advanced healthcare systems, and regulatory support, these factors position Europe as a key growth region for pharmaceutical emulsions, enabling both market expansion and novel therapeutic developments.
Market Segments
The injectable segment leads the pharmaceutical emulsions market, holding 56.13% share in 2024 and growing fastest.
in January 2025, Glenmark Pharmaceuticals Inc., USA, announced the launch of Phytonadione Injectable Emulsion USP, 10 mg/mL Single Dose Ampules. Glenmark's Phytonadione Injectable Emulsion USP, 10 mg/mL Single Dose Ampules is bioequivalent and therapeutically equivalent to the reference listed drug, Vitamin K1 Injectable Emulsion USP, 10 mg/mL of Hospira, Inc.
By Type: (Oil-in-Water (O/W), Water-in-Oil (W/O), and Mixed Emulsions)
By Route of Administration: (Oral, Injectable, Topical / External, Rectal / Vaginal, and Others)
By Application: (Oncology, Cardiovascular, Neurology, Anti-Infectives, Dermatology, and Others)
By Distribution Channel: (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies)
By Regions Covered: (North America, Europe, Asia-Pacific, South America and the Middle East & Africa)
Buy Now & Unlock 360° Market Intelligence:- https://www.datamintelligence.com/buy-now-page?report=pharmaceutical-emulsions-market
Recent Developments:
• In April 2025, Avenacy launched Propofol Injectable Emulsion, USP in the United States as a therapeutic generic equivalent for Diprivan, as approved by the U.S. Food and Drug Administration. Propofol Injectable Emulsion, USP is an intravenous general anesthetic and sedation drug indicated for the induction of general anesthesia for patients greater than or equal to 3 years of age, maintenance of general anesthesia for patients greater than or equal to 2 months of age, initiation and maintenance of monitored anesthesia care (MAC) sedation in adult patients, sedation for adult patients in combination with regional anesthesia, and intensive care unit (ICU) sedation of intubated, mechanically ventilated adult patients
• In January 2025, Glenmark Pharmaceuticals Inc., USA, announced the launch of Phytonadione Injectable Emulsion USP, 10 mg/mL Single Dose Ampules. Glenmark's Phytonadione Injectable Emulsion USP, 10 mg/mL Single Dose Ampules is bioequivalent and therapeutically equivalent to the reference listed drug, Vitamin K1 Injectable Emulsion USP, 10 mg/mL of Hospira, Inc.
• In May 2024, Baxter International Inc. announced US FDA approval of an expanded indication for Clinolipid (Lipid Injectable Emulsion) to be used in pediatric patients, including preterm and term neonates. Clinolipid is Baxter’s proprietary mixed oil lipid emulsion that is used to provide calories and essential fatty acids in parenteral (intravenous) nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated. Clinolipid has been available in the US for adults since 2019 and is now available for use in all ages.
Related Reports:
Pharmaceutical Visual Inspection Systems Market
Pharmaceuticals Market
About Us:
DataM Intelligence 4Market Research is a market intelligence platform that gives access to syndicated, customized reports and consulting to its clients in one place. As a firm with rich experience in research and consulting across multiple domains, we are a one-stop solution that will cater to the needs of clients in key business areas. DataM Intelligence has an online platform whose coverage includes industries such as chemicals and materials, agriculture, health care services, animal feed, and food & beverages among others.
Our platform has Insights on markets that uncover the latest market research data that are distinct from the competition. With coverage across 10 major industries in the marketplace research, DataM Intelligence benefits thousands of companies by helping them take their innovations early to the market, and by providing a complete view of the market with statistical forecasts. Our strategy-centric framework and value-added services will let individuals and corporates with ease of access and custom personalization to research and markets.
Sai Kiran
DataM Intelligence 4Market Research LLP
877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.